Background: The association between immunosuppressant drugs and lymphoma has been reported frequently; however, the precise relationship remains uncertain. Non-Hodgkin lymphoma (NHL) is among the most common cancers associated with long-term immunosuppressant drug use. The FDA Adverse Event Reporting System (FAERS) is a publicly available tool that collects reports of adverse events from healthcare professionals, manufacturers and consumers.
Methods: Nine immunosuppressant drugs belonging to different classes were shortlisted . The FAERS database was searched for all types of NHL as an adverse event reported between 2013 and 2022. Reporting odds ratio (ROR) was used to measure disproportionality in reporting of adverse events for these drugs.
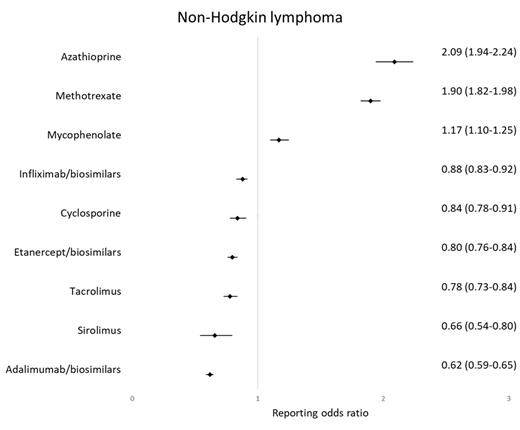
Results: When NHL was analyzed as a percentage of all cancers reported for a particular drug,azathioprine had the highest percentage (22.14%) followed by methotrexate (18.94%), mycophenolate (14.04%), infliximab/biosimilars (11.22%), cyclosporine (10.71%), etanercept (10.44%), tacrolimus (10.13%), adalimumab/biosimilars (8.80%) and sirolimus (8.51%). Drugs with highest ROR for NHL included azathioprine (2.09, 95% CI 1.94-2.24) followed by methotrexate (1.90, 95% CI 1.82-1.98) and azathioprine (1.17, 95% CI 1.10-1.25). Drugs with lowest ROR for NHL included adalimumab/biosimilars (0.62, 95% CI 0.59-0.65) followed by sirolimus (0.66, 95% CI 0.54-0.80) and tacrolimus (0.78, 95% CI 0.73-0.84).
Conclusions: Although all immunosuppressive drugs have been associated with an increased risk of developing NHL, it is advisable to exercise caution with prescribing azathioprine, methotrexate and mycophenolate in patients with preexisting risk. While the use of adalimumab is an independent risk factor for the development of lymphoma, it had the lowest odds of NHL compared to other immunosuppressant drugs in the study. Prospective studies are needed to better understand the true incidence of NHL associated with immunosuppressant drugs.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal